|
In 1851, Gosse had recommended the system for the marine tanks at the new Fish House in the London Zoo, designed so as to not require replenishing, except for adding freshwater to replace that had evaporated; but by 1853, it was proving to be a failure and the Zoological Society of London had to keep buying expensive additional seawater in order to keep the exhibits alive. Then in July of 1853 when Gosse presented his bill for the marine specimens he had collected for the Fish House, the Society’s Council found that Gosse had also collected and sent specimens to the Surrey Zoological Gardens (a competitor to the London Zoo) during his time collecting in Weymouth but charged the Zoological Society for this time. The Council was incensed and angrily denounced his want of consideration for the Society. Gosse was henceforth persona non grata with the Zoological Society of London.

However, there was an opportunity for him to increase his reputation within the scientific community. He had come across a paper written by Edward G. Schweitzer who presented his results in analyzing the waters in the English Channel off the coast of Brighton (Schweitzer, 1839). Schweitzer, was a German physician and chemist, and Director of the Royal German Spa in Brighton. Schweitzer and Warington, by the way, knew each other. A list of the officers and members of the Chemical Society of London dated June 1843 shows Warington as one of its two Secretaries and Schweitzer as one of its members.I’ll explain Schweitzer’s interest by quoting the final paragraph of his paper: |

It is amusing to note that the start of the development of artificial seawater for marine aquaria began

|
Page 2
|
with desire of the Victorians to immerse
themselves in a tub full of water containing bath
salts, and the more genuine or natural the salts the
better! Now there are a number of reasons why aquarists might be interested in artificial seawater rather than using the real thing. The density of the final solution is controllable, it is more stable, the formula can be varied for specific uses (e.g., for marine plants versus for marine animals) and it is sterile and thus diseases cannot spread. In Gosse’s time, however, the main reason was economics. It was a lot less expensive to reconstitute the seawater with local freshwater than to bring seawater to the aquarium. Water is heavy, its salts are not. |
water before Warington did, it would be a very
great advantage in this regard.
Therefore, Gosse devised the following plan. He would visit Warington and steer the conversation in such a way that it would not be a consultation but Warington would talk about his experiences. Executing his plan, Gosse visited Warington in 1854 on two occasions at Apothecaries’ Hall: on January 16th and again on January 21st. As it turns out, however, Warington did consider Gosse’s visits to be consultations. During these “chats,” Warington had no idea that Gosse already knew about Schweitzer’s paper. However, Gosse did discover that Warington had no plans to publish a paper dealing with artificial seawater. Gosse then went ahead with his plan to write his article and by April 21, 1854 he had devised an artificial seawater formula, made up a tank with the solution and added some marine invertebrates. Everything went well and on June 9th he submitted his paper to The Annals and Magazine of Natural History. On July 1954 it was published (Gosse, 1854) and Gosse had achieved his goal. For once, he had beaten Warington to the printed page and went down in history as the first to write about - and to provide a formula for - artificial seawater for aquarists! 
|
Page 3
|
For comparison purposes, Schweitzer had included
the seawater formula based upon a sample
from the Mediterranean Sea devised by G. Laurens.
I wanted to find out something about him
and why he did his analysis but unfortunately,
Gosse had labeled him three times in his paper as
“M. Laurent” (the M standing for Monsieur).
Gosse wasn’t the only one who made this error,
however. In an report made on the action of air
and water on cast iron, wrought iron and steel,
Robert Mallett (1841) also misspelled the name
as “Laurent.” I finally went to Schweitzer’s paper
- something I should have done in the first
place - and found the correct spelling. I also consulted
Laurens’ paper and found out that Gosse
had made errors in Laurens’ calcium sulphate and
calcium carbonate numbers, and had also omitted
entirely Laurens’ entry for magnesium carbonate.
Clearly Gosse had been in too much of a hurry to
get his paper published.
In any event, in the original source (Laurens,
1834) I found that Laurens was a pharmacy student
in Marseille and had sent a note to the Socié-
té de Pharmacie containing an analysis that he
had made of the water of the Mediterranean Sea.
The commentary on the article included the following
(translated): |
From this, there were unexpected, disastrous consequences. One wonders what Warington really thought when he read Gosse’s paper. He must, at the very least, have been dumfounded but as will be seen, he actually only used the word “surprised.”
In any event, Gosse had miscalculated converting
from grains to avoirdupois pounds and ounces,
and had mistakenly assumed that the magnesium
sulphate in Schweitzer’s paper represented the
ordinary crystallized salt, and not the anhydrous
sulphate that is always the case in giving analytical
results. Furthermore, Gosse had eliminated
the following from Schweitzer’s formula:
Gosse’s decision to eliminate magnesium bromide and calcium sulphate simply because Laurens found them absent or in a minute concentration made no sense. The composition of Mediterranean waters differs from that of the water in the English Channel. The English Channel sits on top of layer of chalk and it was to be expected that its calcium content would be higher. Since Schweitzer’s analysis was appropriate for marine animals found off the southern cost of England where most specimens would be collected for British aquaria, it was the relevant analysis and logically the one that should have been followed. |
Page 4
|
Warington responded to Gosse’s paper thusly
(Warington, 1854):
|
In this regard I fault Gosse. Up to this point
Gosse and Warington had been good friends and
Gosse should not have kept Warington in the dark
about what he knew, In any event, the two never
corresponded or met again.
The second reaction was a defense of his formula. At one point Gosse states: Essentially Gosse claimed in his rebuttal that (1) his formula was “…for practical people, to whom minute accuracy is impossible and to whom a chemical formula expressed in quantities of four or five decimals would certainly act as a prohibition,” and (2) by actual experimentation, examples of Crustacea (crabs, etc.), Mollusca (sea snails, etc.), Annelida (worms, etc.) and Zoophyta (sponges, etc.), as well as Ulva and Conferva plants, thrived for over eight months. As can be seen from Table I, the differences between his formula and that of Schweitzer’s were large enough to raise some eyebrows. Ironically, with regard to simplicity it was Warington who suggested the simplest solution of all (Warington, 1854): |
Page 5
Now the major ionic components of seawater are the same as those ions responsible for maintaining the electrical and osmotic balance within the cells of living animals and for the transmission of nerve impulses. What is an ion? Well sometimes atoms gain or lose electrons. The atom then loses or gains a “negative” charge. These atoms are then called ions. For example, when common salt is dissolved the Na (sodium) loses an electron and the Cl (chlorine) gains an electron; the Na becomes Na+ (the sodium ion) and the chlorine becomes Cl- (the chlorine ion). The major ionic components are shown in Table II. The complete absence of any one of these major ions is fatal. A fish placed into an artificial seawater that contained |
no calcium, for example, would soon die
as a result of the chemical imbalance. Therefore,
one of the theoretical problem’s with Gosse’s
formula was that he had omitted the calcium.
Fortunately for Gosse, laboratory grade chemicals
are not 100% pure and technical grade chemicals
are far less so, especially in Gosse’s day. Thus
Gosse’s formulation not only contained the necessary
calcium but it also contained trace elements,
such as silica and iron, that known to be
biologically active.
In support of his assertion that his formula
worked, Gosse provided the following postscript
to his article:
|
| Table 1
THE SCHWEITCHER, GOSSE AND LAURENS FORMULAS COMPARED The numbers are pounds / 100 lbs of water ( = 10 imperial gallons) |
||||
| COMPONENT | SCHWEITZER | GOSSE | % increase in (1) over (2) | LAURENS |
| Sodium Chloride | 2.7060 | 2.1875 | 24% | 2.722 |
| Potassium Chloride | 0.0766 | 0.0563 | 36% | 0.001 |
| Magnesium Chloride | 0.3666 | 0.2813 | 30% | 0.614 |
| Magnesium Bromide | 0.0029 | - | - | - |
| Calcium sulphate | 0.1407 | - | - | 0.015 |
| Magnesium sulphate | 0.2296 | 0.1563 | 47% | 0.702 |
| Calcium carbonate | 0.0030 | - | - | 0.009 |
| Magnesium carbonate | 0.0030 | - | - | 0.011 |
Page 6
|
However, after Gosse had learned of Wilson’s remarks,
he sent Wilson two samples of his artificial
seawater, both of which had sustained living plants
and animals, one for six months and the other for
ten months. By the next year, Wilson had completed
his analyses of Gosse’s samples. The following
is an extract from The Eclectic Magazine, reporting
on the meeting of The British Association for
the Advancement of Science in Glasgow in 1855
(Anon., 1855):

|
Page 7
Wilson, therefore, found trace elements of calcium, phosphorus, silica, iodine and iron in the sample, and offered some ideas on how they arrived there. He neglected, however, to mention the impurities of the constituents and also that trace elements could find their way into the artificial seawater through the foods and the water used to reconstitute the seawater. He did note that most likely other substances, such as bromides, fluorides, ammonia and nitrates, were in the samples but the samples were small, defying detection using the analytical tools available at the time. Aquarists were satisfied by this report coming from a scientific person of such high stature and began to experiment themselves with Gosse’s formula. However, a year later, Hibberd wrote (1856): |
GETS A BIG BOOST In his Hand-Book to the Marine Aquarium (June 23, 1873), Lloyd was still favorably disposed to Gosse’s formula: However, by 1879 Lloyd had changed his mind. He had secured the post of Superintendent of the Aston Aquarium and since it was located far from the coast where seawater was easily obtained and transportation of the water from the coast would be expensive, Lloyd decided that the use of artificial seawater was justified and selected the Schweitzer formula. Thus was the first use of artificial seawater in a public aquarium in England. In an article titled, “The Artificial Sea-Water at the Aston Aquarium”, William Southall (1879) wrote:
|
Page 8
|
of Birmingham. Above the dotted line are the concentrations
of the salts added to the well-water (as
can be seen, the components and concentrations
are essentially identical to those of the Schweitzer
formula) and below are the components and concentrations
of what was found in an analysis of the
well water. Added together they are the actual
components and concentrations in the Aston tanks.
It is clear from these measurements that the water added to a basic group of components is a major consideration with regard to trace elements. All freshwater contains one or more of those used in the Aston Aquarium, more or less, but usually more. Lloyd pointed out that the Thames at London contains about 0.02 or 0.03 pounds of trace elements instead of the roughly 0.01 lbs. per 100 lbs. of water of the Aston well water (Lloyd,1879). Considering the accuracy required for the correct |
reproduction of certain of the minor constituents,
the purity of the added salts and of the water used
is critical, endangering the final formulation unless
the quantity and quality of the impurities are
known. For example, if the sodium chloride used
is the commercial grade or household salt, the
arsenic and barium levels (even though these are
in minute quantities and can be measured in single
figures in parts per million) can present problems
unless accounted for. In addition to these
two, sodium chloride of this grade contains as
many as twelve other elements in substantial
enough quantities to give rise to doubt as to what
their final concentrations would be.
|
|
TABLE III THE ASTON FORMULA COMPARED TO THE SCHWEITZER FORUMULA The numbers are pounds / 100 lbs. of water (= 10 imperial gallons) |
||
| COMPONENT | SCHWEITZER | ASTON |
| Sodium Chloride | 2.70595 | 2.72143 |
| Potassium Chloride | 0.07655 | 0.07857 |
| Magnesium Chloride | 0.36666 | 0.37643 |
| Magnesium Bromide | 0.00293 | 0.00400 |
| Calcium Sulphate | 0.14063 | 0.14429 |
| Magnesium Sulphate | 0.29578 | 0.23700 |
| Calcium Carbonate | 0.00330 | 0.00841 |
| Calcium Carbonate | 0.00329 | |
| Magnesium carbonate | 0.00217 | |
| Silica | 0.00148 | |
| Magnesium sulphate | 0.00046 | |
| Oxide of iron and alumina | 0.00022 | |
| Sodium Chloride | 0.00201 | |
| Magnesium nitrate | 0.00050 | |
| Sodium nitrate | 0.00201 | |
| Potassium chloride | 0.00006 | |
| Sodium iodide | traces | |
| Ammoniacal salts & organic matter | slight traces | |
| Those below the dashed line were present in the well water. | ||
Page 9

The 1870s voyage of H.M.S. Challenger lasted 1,000 days and covered more than 68,000 nautical miles. Many consider it to be the first true oceanographic expedition because it yielded a wealth of information about the marine environment. Those aboard identified many organisms then new to science, and they gathered data at 362 oceanographic |
stations on temperature, currents, water chemistry
and ocean floor deposits. The scientific results of
the voyage were published in a 50-volume, 29,500-
page report that took 23 years to compile. Specialists
in numerous scientific disciplines studied the
collections and data, and helped produce the reports.
Also, the reports written by members of the Challenger expedition provided rich descriptions of the flora, fauna and cultures of the lands visited. Photography - new at the time - was highlighted as well, along with scientific illustration. The HMS Challenger originally was designed as a British warship - a steam corvette in the Royal Navy - outfitted with 17 guns and an engine capable of over 1,200 horsepower. The 200-foot ship was three-masted, square-rigged and built of wood. In 1870, Dr. Charles Wyville Thomson, a Scottish natural historian and marine zoologist, suggested that the Royal Society of London ask the British government for the use of one of its ships for an extended research cruise. The government agreed, and the HMS Challenger was modified to conduct oceanic research. Ammunition and 15 of the guns were removed from the ship and replaced with laboratories, workrooms, and storage space. The HMS Challenger used sails rather than the steam engine most of the time to allow for frequent stops when collecting data. The steam engine was used only during dredging operations to collect samples from the depths of the ocean. At the start of the voyage in 1872, the science and ship crew consisted of six civilian/scientific staff, led by Dr. Thomson. It also included 21 naval officers, including 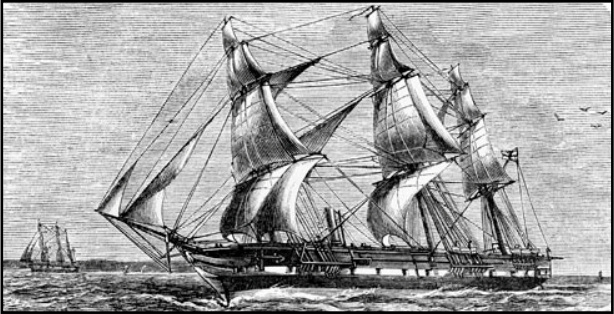
|
Page 10

|
|
Page 11

It should be noted that the analysis of seawater is primarily concerned with its ions, notably chloride, sodium, sulphate, magnesium, calcium and potassium. To construct a formula useful for the manufacture of artificial seawater, however, this information must be translated into specific chemical compounds. This is an arbitrary procedure since it doesn’t make any difference what chemical compounds and their concentrations are chosen as long as the mixture after being dissolved contains the ions in their correct amounts. An example is potassium. Schweitzer added it as potassium chloride and adjusted the amount of chloride elsewhere; Dittmar added it as the sulphate and adjusted the amount of sulphate elsewhere. Thus, combining the acids and bases in the arbitrary mode shown in Table IV, Dittmar held that the seven numbers in the table expressed as percentages of the total salts - |
Column (1) - may be taken as holding approximately
for any sample of seawater, in other words,
the ratios of these components is constant. I quote
Dittmar here:
In order to derive a formula for artificial seawater, one has to know the salinity, i.e., the total amount of the salts in the water. For Table IV, I chose the average ocean salinity of 3.5 grams of salts per 100 grams of seawater. By multiplying the percentage figures in Column (1) of Table IV by this number, I obtained the gram weights for each compound; these are listed in Column (2) as grams per 100 grams of seawater. As Warington showed in his critique of Gosse’s paper, we can consider these numbers as lbs./100 lbs. of water and the Dittmar and Schweitzer formulas, therefore, can be compared directly in Table IV. There are some significant differences, mainly in the magnesium sulfate, calcium carbonate, and magnesium bromide. |
Page 12
| If Dittmar’s formula may have been of some interest to public aquaria, it had no effect on the aquarium hobby. The interest in marine aquaria had waned because it was expensive and required careful attention on the part of the hobbyist. In this country Stage IV of the aquarium hobby (the Stage we are in now) started just before 1900, but the emphasis was on freshwater and, to a very limited extent, brackish water fishes. Some public aquaria, particularly those situated inland, did use artificial seawater but this had no real effect on the hobby until after World War II ended. This did not , however, stop oceanographers from devising new formulas, such as McClendon et. al in 1917 and Brujewicz (in Subow, 1931). Furthermore, everytime the International Atomic Weights were revised, the formulas were revised also. In 1940, for example, |
Lyman and Fleming recalculated Dittmar’s figures
using the 1938 Atomic Weights.
The Lilly Ackerland Fleischmann Memorial Aquarium at the Cincinnati Zoo opened on Friday, May 26, 1950. I moved to Cincinnati in June of 1950 and by 1951 I was a consultant to the Aquarium, mostly engaged in identifying species of newly-obtained fishes. The Aquarium was interested in displaying marine life - it was all freshwater at the time - and wanted to set up some experimental marine aquaria. However, at that time there were no artificial seawater mixes on the market, so I was asked to provide a formula. In early 1952 I turned over a copy of what I had come up with to the Chief Aquarist and we sat down for a lengthy discussion over the details. It was decided to go ahead with the experiments and |
THE SCHWEITZER AND DITTMAR FORMULAS COMPARED
|
|||
COMPONENT |
(1) SALT % |
(2) DITTMAR |
(3) SCHWEITZER |
| Sodium Chloride | 77.758 | 2.7215 | 2.7060 |
| Magnesium chloride | 10.878 | 0.3807 | 0.3666 |
| Magnesium sulphate | |||
| Calcium sulphate | |||
| Potassium Chloride | 0.0766 | ||
| Potassium sulphate | 2.465 | 0.0863 | |
| Magnesium bromide | 0.217 | 0.0076 | .0029 |
| Calcium carbonate | 0.345 | 0.0121 | 0.0030 |
| TOTAL | 100% | ||
Page 13
|
so the Aquarium had a quantity of the mixture
made up by a local commercial chemist, enough
for about 100 gallons of water. In May of that year,
however, I entered the United States Army (the
Korean War still was in full tilt) so I was not
around to see how things turned out. The following,
however, is from the Cincinnati Enquirer of
Sunday, November 16, 1952:
The caption under one of the pictures read: After my tour of duty ended (I had been Commanding Officer of the 501st Chemical Depot Company), I spent a year in graduate school and returned to Cincinnati at the end of August 1955. I renewed my acquaintances with the Cincinnati Zoo and Fleischmann Aquarium staff and was able to examine the seven tanks that utilized the formula that I had provided. Everything was fine; the vertebrates, were in good health, as well as were the anemones and other marine creatures. The Lilly Ackerland Fleischmann Memorial Aquarium at the Cincinnati Zoo.

|
Much to my surprise, I learned that during my absence
the Aquarium had decided to sell the salt
mixture to aquarists under the name of “Marine
Mix.” This was done mainly because it was cheaper
to manufacture in large quantities than small.
Thus, in 1956 the first artificial seawater mix appeared
on the market in this country. It was only
on the market briefly since the Fleischmann
Aquarium was satisfied that artificial seawater was
feasible and so they planned to build an East Wing
on the building to house small marine tanks. This
took some time to plan, secure the necessary funds
and build, but the addition was finally opened in
June 1972. By this time, of course, there were a
number of improved mixes on the market and the
Aquarium did not need to manufacture its own as it
did in the 1950s.
THE COMPOSITION OF MARINE MIX The element strontium, one of the major constituents of seawater, had not been isolated in Dittmar’s time. The usual wet-chemical analysis methods for calcium register any strontium present as an equivalent amount of calcium As a result, the strontium went unreported in the early analysis of seawater. I had briefly looked over several older formulas, but settled on that of Lyman and Fleming (1940) since they had included strontium. Their formula yields a water containing all of the major ionic components in their correct proportions, lacking only in the minor elements. First, some biographical information. Dr. John Lyman (1915-1977) directed studies of the ocean for three agencies in the course of 20 years with the federal government. Born in Berkeley, Calif., Lyman did undergraduate work there and received his master's and doctorate degrees from Scripps Institution of Oceanography, part of the University of California in Los Angeles. He began his career in Washington in 1946, when he became director of oceanography for the Navy Hydrographic Office. He later became director of oceanography at the National Science Foundation and then chief adviser for oceanographic research at the Bureau |
Page 14

Richard Howell Fleming (1909-1989), was a Canadian-born American oceanographer who conducted wide-ranging studies in the areas of chemical and biochemical oceanography, ocean currents (particularly those off the Pacific coast of Central America), and naval uses of oceanography. He joined the Scripps Institution in La Jolla, California, in 1931 and served as assistant director from 1946 to 1950, when he left to become professor of oceanography at the University of Washington. Fleming measured the amounts of chemical elements in sea water and in marine life. His work on ocean currents included measurement of tidal current velocities, description of upwelling of seawater, and observations of seasonal variations in currents. He also explored sedimentation in moving |
and stagnant bodies of water. With H.U.
Sverdrup and Martin W. Johnson, Fleming wrote
The Oceans (1942).
Those aquarists not interested in an arithmetic exercise can safely skip this part of the Addendum and go directly to Column (2) of Table V. Referring to Table V, lines 1-10 in Column (1) comprise the Lyman and Fleming formula; lines 11 -17 are my additions. The sum of lines 1-10 in Column (1) is 34.481 grams. This, by the way, rounded is a salinity of 34.5 (in grams of salts per 1000 grams of seawater). Subtracting this from 1000 grams (1 kilogram) we obtain 965.519 grams of water. (Items 11-17 were not included since they are in quantities too small to have any effect on these calculations.) Since 1 gallon of water = 3.79 kilograms, 965.519/3790 = 0.2547543 gallons. (At this stage in the computations I like to keep as many decimal places as possible in order to minimize rounding errors.) Now I wanted a formula for 10 gallons of water, not 0.2547543 gallons. Therefore, I multiplied the lines 1-17 in Column (1) by 10/0.2547543 = 39.253508 and obtained the final formula for Marine Mix shown in Column (2). The salinity of this formulation was not affected and remained at 34.5. There has always been controversy over the relative merits of natural and synthetic seawater, especially with regard to trace or minor elements. Most synthetic seawaters have at some stage failed in

|
Page 15
|
certain respects, such as in maintaining a specific
marine organism or plant, or in the breeding and
rearing of the higher forms of marine life such as
the fishes. A list of components in seawater of
proven or suspected biological activity, in order of
their concentrations in micrograms per liter (μ/l), is
shown in Table VI. Copper, Barium and Nitrate (as
NO3) are found in Cincinnati water. If I had such a
list when I was putting together my formula for
Marine Mix, I might have replaced the Barium,
Aluminum and Copper with Silicon, Iron and Molybdenum.
Anon., “A List of the Officers and Members of the Chemical Society of London. June 1843,” The Chemical Society of London, Richard and John E. Taylor, London, 1843. Anon., “Scientific Pickings from The British Association Papers,” The Eclectic Magazine, p. 1071, December, 1855. |
|
COMPONENT |
(1) AMT. 1/KG |
(2) AMT ADDED TO 10 GAL. |
| 1. Sodium chloride, NaCl | 23.476 grams | 921.515 grams |
| 2. Magnesium Chloride, MgCl2 | 4.981 | 195.522 |
| 3. Sodium sulphate, Na2SO4 | 3.917 | 153.756 |
| 4. Calcium Chloride, CaCl2 | 1.102 | 43.257 |
| 5. Potassium chloride, KCl | 0.664 | 26.064 |
| 6. Sodium bicarbonate, NaHCO3 | 0.192 | 7.367 |
| 7. Potassium bromide, KBr | 0.096 | 3.768 |
| 8. Boric acid, H3BO3 | 0.026 | 1.021 |
| 9. Strontium chloride, SrCl2 | 0.024 | 0.942 |
| 10. Sodium Flouride, NaF | 0.003 | 0.118 |
| 11. Disodium phosphate, Na2HPO4 | 0.250 milligrams | 9.813 milligrams |
| 12. Potassium iodide, (KI) | 0.077 | 3.023 |
| 13. Alum, (AlK(SO4) | 0.044 | 0.727 |
| 14. Zinc sulphate, ZnSO4.7H2O | 0.015 | 0.589 |
| 15. Manganese chloride, MnCl2.4H2O | 0.005 | 0.186 |
| 16. Barium chloride, BaCl2.2H2O | 0.005 | 0.196 |
| 17. Copper sulphate, CuSo4.5H2O | 0.002 | 0.076 |
| 18. Water | 65.519 grams | 10 gallons |
Page 16
|
Dittmar, Wilhelm, “Report on Researches into
the Composition of Ocean-Water, collected by
H.M.S. Challenger, During the Years 1873-1876,”
in Report on the Scientific Results of the Voyage
of H.M.S. Challenger During the Years 1873-76,
Physics and Chemistry - Vol I., Received January
1884.
Forchhammer, George, “On the Constitution of Sea-Water, at Different Depths, and in Different Latitudes,” Proceedings of the Royal Society of London, Vol. 12, pp. 129-132, 1862-1863. Gosse, Philip H., “On keeping Marine Animals and Plants alive in unchanged Sea-water,” The Annals and Magazine of Natural History, Vol. X, Second Series, pp. 263-268, October, 1852. Gosse, Philip H., “On Manufactured Seawater for the Aquarium,” The Annals and Magazine of Natural History, Vol. 14, Second Series, 65- 67, 1854. Gosse, Philip H., “On Artificial Sea Water ,” Annals and Magazine of Natural History, Vol. XV, Second Series, No. 85, pp. 17-19, 1855. Hibberd, Shirley, “The Book of the Aquarium and Water Cabinet,” Groombridge & Sons, London, pp. 59-60, 1856. Lloyd, William Alford, “Sea-Water for Aquaria,” The Boy’s Own Paper, No. 49, Vol. II, p. 190, Saturday, December 20, 1879. Lyman, John and Fleming, Richard Howell, “Composition of Sea water,” Journal of Marine Research, vol. 3, pp. 134-146, 1940. Mallet, Robert, “Second Report upon the Action of Air and Water, whether fresh or salt, clear or foul, and at various temperatures, upon Cast Iron, Wrought Iron, and Steel,” Report of the Tenth Meeting of the British Association for the Advancement of Science held at Glasgow in August 1840, p. 223, John Murray, London, 1841. McClendon, J. F., C. C. Gault, and S. Mulholland, “The hydrogen-ion concentration, CO2− tension, and CO2−content of sea water,” Carnegie Institute of Washington, Publication no. 251, Papers from the Department of Marine Biology, pp. 21-69, 1917. |
Schweitzer, Edward G., “Analysis of Seawater
as it exists in the English Channel near Brighton,”
Philosophical Magazine and Journal, XV, pp. 51-
60, 1839
Schweitzer, Edward G., “Analysis of the Bonnington Water, near Leith, Scotland,” Memoirs and Proceedings of the Chemical Society of London, II, pp. 201-217, 1843. Southall, William, “The Artificial Sea-Water at the Aston Aquarium,” Read before the Birmingham Natural History and Microscopial Society, September 23, 1879, The Midland Naturalist, Volume II, p. 246-247, 1879. Subow, N. N., “Oceanographical tables. U.S.S.R.” , Oceanographic Institute, Hydrometeorological Commission, 208 pp., Moscow, 1931. Warington, Robert, “Notice of Observations on the adjustment of the relationship between the Animal and Vegetable Kingdoms, by which the vital functions of both are permanently maintained.” A paper read before the assembled members of the Chemical Society on the evening of the 4th March, 1850. Warington, Robert, “The Aquatic Plant Case, or Parlour Aquarium, “The Garden Companion, and Florists’ Guide, William S. Orr and Co., Amen Corner, Paternoster Row, p. 7, January, 1852. Warington, Robert, “On preserving the balance between the animal and vegetable organisms in sea -water,” The Annals and Magazine of Natural History, Second Series, Vol. 12, No. 71, pp. 319-324, 1853. Warington, Robert, “On Artificial Sea Water,” The Annals and Magazine of Natural History, Vol. 14, No. 84, pp. 419-421, 1854. Wilson, George, “On the Artificial Preparation of Sea-water for Marine Aquaria,” Report of the Twenty-Fourth Meeting of the British Association for the Advancement of Science, Notices and Abstracts of Miscellaneous Communications to the Sections, p. 77, Liverpool, September, 1854. |